Check out our latest products
Researchers from the Institute of Science Tokyo have discovered a rubidium-based material that conducts well and stays stable, which could help make solid oxide fuel cells work better.
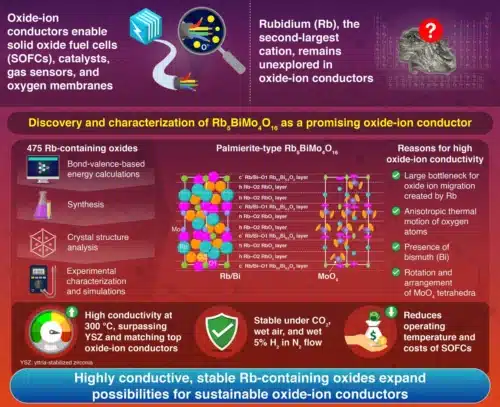
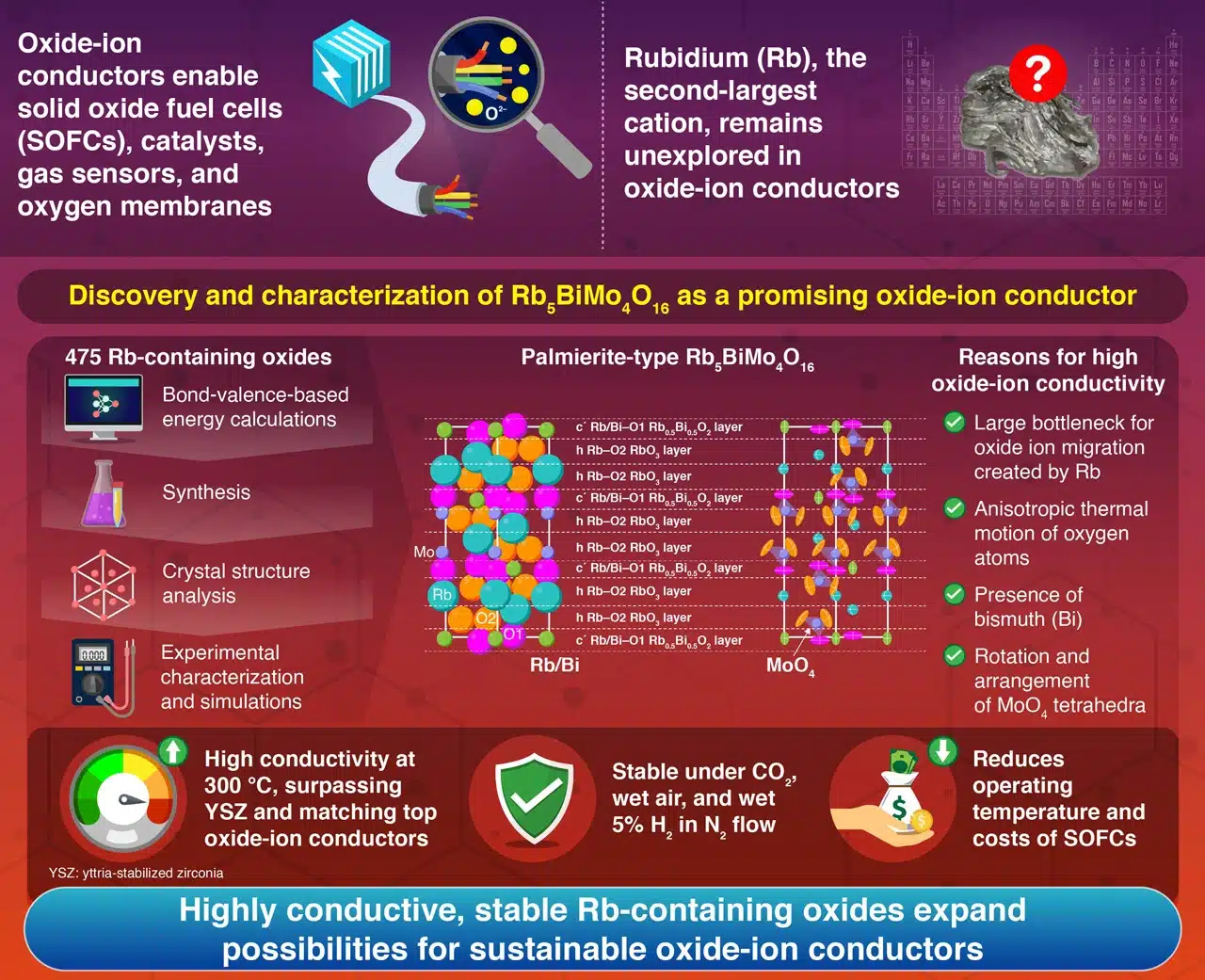
Oxide-ion conductors enable oxide ion (O²⁻) transport in solid oxide fuel cells (SOFCs), allowing them to operate on fuels beyond hydrogen, such as natural gas, biogas, and certain liquid hydrocarbons, making them valuable during the transition to a hydrogen-based energy system. However, cost, durability, and operating temperature challenges limit their widespread adoption, driving researchers to explore new oxide-ion conductor materials. One potential breakthrough element is rubidium (Rb), which a research team from the Institute of Science Tokyo systematically investigated for its potential role in high-performance oxide-ion conductors.
Since Rb⁺ is among the largest cations, Rb-containing oxides are expected to have expanded lattice structures and larger free volumes, which could reduce activation energy for oxide-ion transport. The researchers tested 475 Rb-containing oxides using bond-valence-based energy calculations to test this. They identified palmierite-type oxides, which share structural similarities with the naturally occurring mineral palmierite, as promising candidates due to their relatively low energy barrier for oxide-ion migration.

Building on past research showing high oxide-ion conductivity in bismuth (Bi)- and molybdenum (Mo)-containing materials, the team focused on Rb₅BiMo₄O₁₆. They conducted material synthesis, conductivity measurements, stability tests, and structural analyses to validate its potential.
Several factors contributed to this high conductivity: the large Rb atoms helped lower activation energy, the MoO₄ tetrahedra’s rotation and arrangement enhanced ion transport, and the anisotropic thermal vibration of oxygen atoms further facilitated conductivity. Additionally, large Bi cations with lone electron pairs reduced activation energy for oxide-ion migration. Beyond high conductivity, Rb₅BiMo₄O₁₆ demonstrated remarkable stability under various conditions, including CO₂ flow, wet air flow, wet 5% hydrogen in nitrogen flow, and even immersion in water at room temperature.
Future research in this area could lead to improved oxide-ion conductors for sustainable energy applications and devices like oxygen membranes, gas sensors, and catalysts.


![[5G & 2.4G] Indoor/Outdoor Security Camera for Home, Baby/Elder/Dog/Pet Camera with Phone App, Wi-Fi Camera w/Spotlight, Color Night Vision, 2-Way Audio, 24/7, SD/Cloud Storage, Work w/Alexa, 2Pack](https://m.media-amazon.com/images/I/71gzKbvCrrL._AC_SL1500_.jpg)



![[3 Pack] Sport Bands Compatible with Fitbit Charge 5 Bands Women Men, Adjustable Soft Silicone Charge 5 Wristband Strap for Fitbit Charge 5, Large](https://m.media-amazon.com/images/I/61Tqj4Sz2rL._AC_SL1500_.jpg)