Check out our latest products
Using everyday materials and a novel solar-powered process, a University of Alberta researcher could revolutionize hydrogen production—potentially leapfrogging current solar technologies.
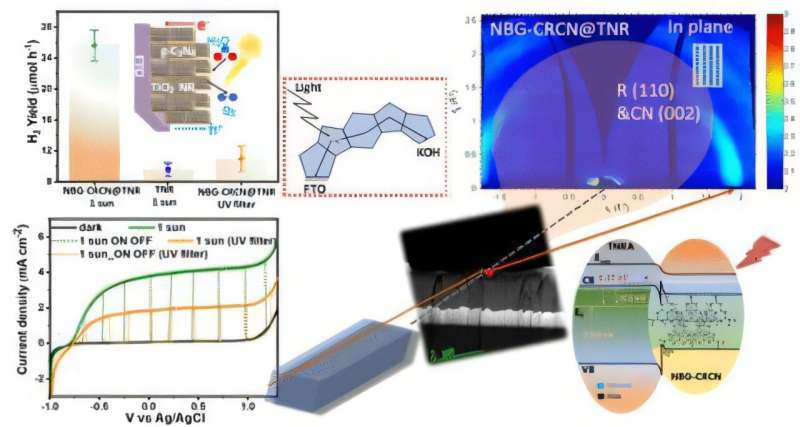
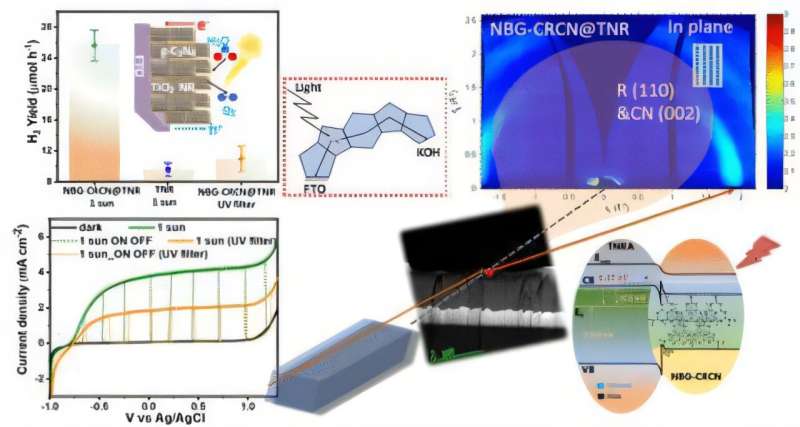
A University of Alberta engineering team, in collaboration with the Technical University of Munich, developed method to produce hydrogen fuel using only sunlight and earth-abundant materials. The research, led by electrical and computer engineering professor Karthik Shankar, was recently published in the Journal of the American Chemical Society.
The team has developed a photocatalytic process using carbon nitride, a material derived from common chemicals like urea and melamine, to split water into hydrogen and oxygen. Unlike traditional solar energy systems that use photovoltaics to generate electricity followed by electrolysis to extract hydrogen—a process that loses significant energy—Shankar’s method combines both steps into one efficient, direct conversion.

“Instead of generating electricity and then using it to split water, we directly harness sunlight to do the job,” explains Shankar. The key lies in creating a junction between carbon nitride and titanium dioxide, forming what’s known as a semiconductor heterojunction. This structure effectively prevents the recombination of electron-hole pairs created by sunlight exposure, allowing them to drive chemical reactions that split water molecules.
The process is powered by carbon nitride’s ability to absorb diffuse sunlight—even on cloudy days—thanks to nanowire surface structures that capture light from multiple angles. The added benefit: titanium dioxide is also inexpensive and abundant, making the technology scalable and economically viable.
Beyond efficiency, the innovation addresses limitations of traditional solar panels, which are intermittent, inefficient under indirect light, and require costly silicon-based manufacturing with significant environmental impacts. “Silicon solar cells dominate the market, but they rely on energy-intensive processes and centralized production in geopolitically sensitive regions,” notes Shankar.
Hydrogen produced this way can also be stored and transported more easily than solar electricity, acting as a dense, portable energy source. Thanks to carbon nitride’s durability and resilience to high temperatures and chemical exposure, Shankar believes the technology could be commercially viable within three to five years—potentially redefining the clean energy landscape.
References: Narendra Chaulagain et al, Heteroepitaxial Growth of Narrow Band Gap Carbon-Rich Carbon Nitride Using In Situ Polymerization to Empower Sunlight-Driven Photoelectrochemical Water Splitting, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c01824 Journal information: Journal of the American Chemical Society


![[5G & 2.4G] Indoor/Outdoor Security Camera for Home, Baby/Elder/Dog/Pet Camera with Phone App, Wi-Fi Camera w/Spotlight, Color Night Vision, 2-Way Audio, 24/7, SD/Cloud Storage, Work w/Alexa, 2Pack](https://m.media-amazon.com/images/I/71gzKbvCrrL._AC_SL1500_.jpg)



![[3 Pack] Sport Bands Compatible with Fitbit Charge 5 Bands Women Men, Adjustable Soft Silicone Charge 5 Wristband Strap for Fitbit Charge 5, Large](https://m.media-amazon.com/images/I/61Tqj4Sz2rL._AC_SL1500_.jpg)