Check out our latest products
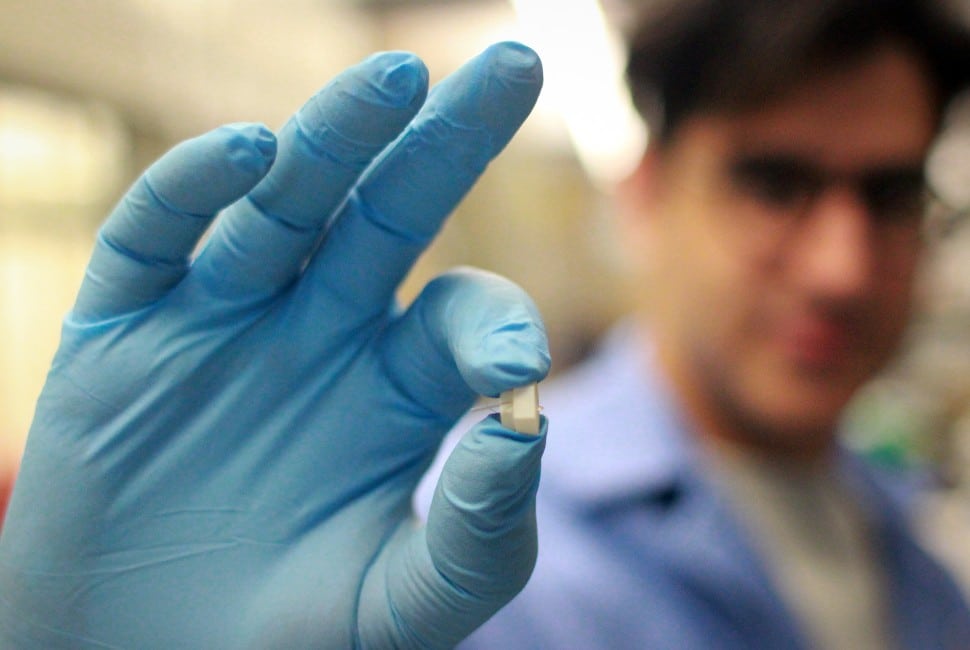
Scientists at Northwestern University have developed an implantable device capable of real-time monitoring of protein levels, enabling tracking of inflammation markers for better disease management.

Northwestern University researchers have discovered a first-of-its-kind sensor that continuously monitors protein fluctuations linked to inflammation within the body. This innovation offers potential in managing acute and chronic illnesses by tracking key proteins like cytokines and heart failure biomarkers. The device is expected to benefit patients with conditions such as diabetes, heart failure, and autoimmune disorders, offering personalised insights to clinicians for tailored treatments.
The device employs DNA strands that alternately bind and release proteins, mimicking the action of tree branches shedding fruit. “This is a completely new capability — to be able to watch inflammation in real time,” explained Shana O. Kelley, the study’s lead researcher. Such functionality could transform how conditions are managed by detecting early warning signs and allowing for timely medical interventions, particularly for individuals who require continuous health monitoring.

The team tested the sensor in diabetic rats, using a microneedle-sized implant that samples fluids beneath the skin. This device demonstrated exceptional sensitivity, accurately detecting cytokine levels as they fluctuated due to fasting, insulin injections, or immune system activation. Remarkably, even minor inflammation from insulin injections was detected, aligning closely with standard laboratory methods.
Inspiration for the sensor came from nature. Postdoctoral fellow Hossein Zargartalebi likened its operation to shaking an apple tree to release fruit. “What if we could ‘shake’ the DNA receptors on our sensors to release proteins similarly?” he recalled. His innovative use of alternating electric fields successfully reset the sensors, enabling continuous real-time monitoring.
The technology holds vast potential beyond inflammation tracking. Kelley highlighted its possible application in monitoring heart failure by continuously tracking BNP protein levels, which could help fine-tune treatments before symptoms worsen. “This could be the ultimate preventative measure,” she noted, likening its impact to the revolutionary benefits of continuous glucose monitoring for diabetes.
This pioneering work sets the stage for advancements in personalised medicine and preventative healthcare.


![[5G & 2.4G] Indoor/Outdoor Security Camera for Home, Baby/Elder/Dog/Pet Camera with Phone App, Wi-Fi Camera w/Spotlight, Color Night Vision, 2-Way Audio, 24/7, SD/Cloud Storage, Work w/Alexa, 2Pack](https://m.media-amazon.com/images/I/71gzKbvCrrL._AC_SL1500_.jpg)



![[3 Pack] Sport Bands Compatible with Fitbit Charge 5 Bands Women Men, Adjustable Soft Silicone Charge 5 Wristband Strap for Fitbit Charge 5, Large](https://m.media-amazon.com/images/I/61Tqj4Sz2rL._AC_SL1500_.jpg)